- Elements
The Energies Listed are Binding Energies!
O 1s: 530 eV
O 2s: 20 eV
O 2p: 8 eV
The Energies Listed are Binding Energies!
Overlaps for O 1s (primary emission)
- Na KLL (Al kα X-rays) (500 eV)
- V 2p (512 eV)
- Dy MNN (Al kα X-rays) (517 eV)
- Sb 3d (528 eV)
- Pd 3d (531 eV)
Energies listed are Kinetic Energies!
O KLL: ~ 505 eV
The Energies Listed are Binding Energies!
| Species | Binding energy / eV | Charge Ref | Ref |
| Ag2CO3 | 530.7 ± 0.1 | C (285 eV) | Ref |
| Ag2O | 529.3 ± 0.1 & 531.1 ± 0.1 | C (285 eV) | Ref |
| AgO | 528.6 ± 0.1 & 530.7 ± 0.1 | C (285 eV) | Ref |
| Al2O3 | 531 ± 0.1 | C (284.8 eV) | Ref |
| AlO(OH) | 531.2 ± 0.1 | C (284.8 eV) | Ref |
| Al(OH)3 | 531.2 ± 0.2 | C (285 eV) | Ref |
| AmOx | 529 | Pt 4f (71.3 eV) | Ref |
| As2O3 | 531.8 ± 0.25 | Au (74 eV) | Ref |
| Au2O3 | 530.2 | Au (74 eV) | Ref |
| Ba(OH)2 | 531.2 | Au (74 eV) | Ref |
| BaCO3 | 530.95 ± 0.15 | C (284.8 eV) | Ref |
| BaO | 530.8 ± 0.2 | (284.8 eV) | Ref |
| BaO2 | 530.8 | C (284.8 eV) | Ref |
| BeO | 531.6 ± 0.1 | C (285 eV) | Ref |
| Bi2O3 | 530.1 ± 0.2 | C (284.8 eV) | Ref |
| Li2O | 530.6 | Au 4f (83.8 eV) | Ref |
| LiOH | 533.6 | Au 4f (83.8 eV) | Ref |
Oxygen spectra of metal oxide systems ranges from simple to highly complex, particularly in the case of mixed metal oxide systems. Often times the dominant signal may be attributed to the lattice oxygen – for simple systems such as SiO2 or TiO2 (Figure 1). These tend to be accompanied by a secondary signal at higher binding energy associated with surface hydroxyls.

Figure 1: Lattice O and surface hydroxyls of P-25 TiO2 and SiO2 nanospheres.1
Appreciation of the lattice peak relative to the hydroxyl may in som cases be tricky, with real surfaces rarely presenting themselves free from surface terminal groups. CryoXPS of liquid metal oxides however, presents the capability to isolate the M-O species free from additional complications, for the right system (Figure 2).
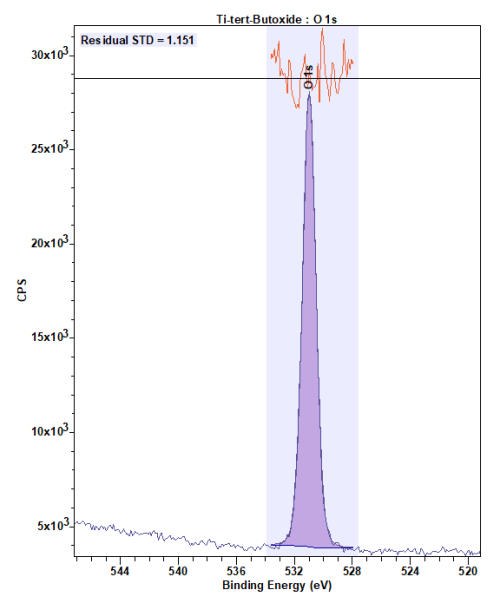
Figure 2: CryoXPS of Titanium tert-Butoxide1,2
Oxygen analysis of mixed metal oxides may prove advantageous when considering quantification analysis of inhomogeneous metal oxides – for example titania monolayers grown onto silica supports,3 since the two species have comparable kinetic energies rendering differing escape depths (of the Ti 2p and Si 2p core lines) a redundant factor when considering surface composition (Figure 3).

Figure 3: O 1s region for a Ti oxide overlayer on Si oxide support.1
Surface basicity
Analysis of the oxygen auger region may yield crucial information regarding surface basicity, particularly crucial when considering solid base catalysts. The auger parameter provides insight into the surface polarizability of the solid material, and hence may be correlated quantifiably with surface basicity (Figure 4).4
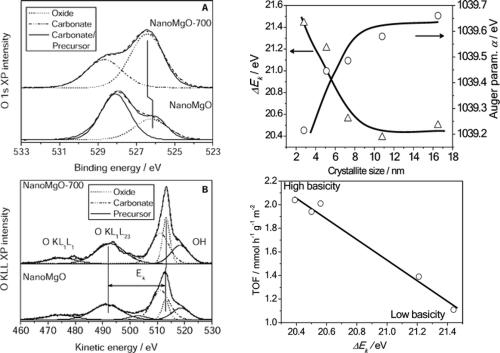
Figure 4: Correlating O 1s – KLL auger parameter with surface basicity.4
References
- Data acquired by HarwellXPS
- Isaacs, Mark A. “Low temperature XPS of sensitive molecules: Titanium butoxide photoelectron spectra.” Applied Surface Science Advances 18 (2023): 100467. Read it online here.
- Isaacs, Mark A., Brunella Barbero, Lee J. Durndell, Anthony C. Hilton, Luca Olivi, Christopher MA Parlett, Karen Wilson, and Adam F. Lee. “Tunable silver-functionalized porous frameworks for antibacterial applications.” Antibiotics 7, no. 3 (2018): 55. Read it online here.
- Montero, Janine M., Pratibha Gai, Karen Wilson, and Adam F. Lee. “Structure-sensitive biodiesel synthesis over MgO nanocrystals.” Green chemistry 11, no. 2 (2009): 265-268. Read it online here.