XPS for Beginners
Fundamentals 1
- Fundamentals of XPS
- Data Analysis
- Applications of XPS
- Related Techniques
- While core orbitals all have their general place in the overall spectrum – small changes to binding energy may be observed due to changes to the nature of the emitting atom
- We can model these changes to determine the chemical nature of our surfaces
- One obvious way in which an atom may change is by changing oxidation state
- If a metal centre transitions from it’s zero-valent form to it’s ionized form upon complexation with an anion/ligand it will obtain an overall positive charge
- Since electron density has been removed, the nucleus will now exert a greater pull upon the remaining electrons – effectively increasing the binding energy
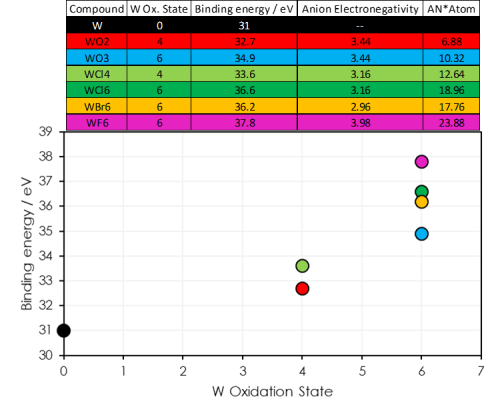
- As well as oxidation state, the neighbouring atoms play a role in determining the binding energy of a core orbital
- Tungsten atoms with the same oxidation state may show differing binding energies
- Electronegative ions withdraw electron density from the target atom – producing a positive dipole and increasing the binding energy of the core orbital
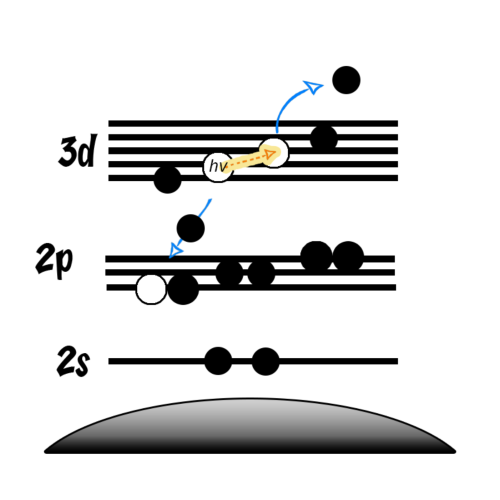
- Auger peaks are secondary electron emissions
- Following a photoemission, electrons in higher energy orbitals may relax to fill the newly created core-hole
- This relaxation releases energy in the form of X-rays
- These X-rays may then excite another electron to the point of photoemission
- Due to the nature of Auger electrons, they tend to be more affected by changes to the valence structure (i.e. bonding) than core orbitals
- One way this can be of use is the Auger parameter
- This involves subtracting the kinetic energy of the related core orbital from that of the auger emission
α = EK(Auger) – EK(Core)
- This was later developed into the modified Auger parameter in order to be independent of excitation energy
- The parameter may be used to probe oxidation states where core level spectroscopy cannot
α’ = EK(Auger) + EB(Core)
Pt (IV)
Correct!
Binding energy scales with oxidation state (with a few exceptions), so in order of increasing binding energy goes: Pt(0), Pt(II) and Pt(IV).
Pt (II)
Incorrect
Binding energy scales with oxidation state (with a few exceptions), so in order of increasing binding energy goes: Pt(0), Pt(II) and Pt(IV).
Pt (0)
Incorrect!
Binding energy scales with oxidation state (with a few exceptions), so in order of increasing binding energy goes: Pt(0), Pt(II) and Pt(IV).
NbO
Correct!N
- NbO BE ~ 203.7 eV
- NbO2 BE ~ 206.2 eV
- Nb2O5 BE ~ 207.4 eV
Nb2O5
Incorrect
- NbO BE ~ 203.7 eV
- NbO2 BE ~ 206.2 eV
- Nb2O5 BE ~ 207.4 eV
NbO2
Incorrect
- NbO BE ~ 203.7 eV
- NbO2 BE ~ 206.2 eV
- Nb2O5 BE ~ 207.4 eV
Al X-rays
Correct!
Remember:
KE = hv – BE – Φ
So, increase energy of X-rays (hv), increase the outgoing kinetic energy!
Mg X-rays
Incorrect
Remember:
KE = hv – BE – Φ
So, increase energy of X-rays (hv), increase the outgoing kinetic energy!