Multiplet Splitting
Multiplet splitting (also termed exchange splitting) is a final state effect which, similar to spin-orbit splitting arises because of interactions between magnetic fields set up by localised spinning charges.
Multiplet splitting arise from:
a: Unparied electrons remaining in the core-level containing the core-hole
b: Unpaired valence electrons, whether initially present or introduced by relaxation (note these become localised by the newly formed core-hole)
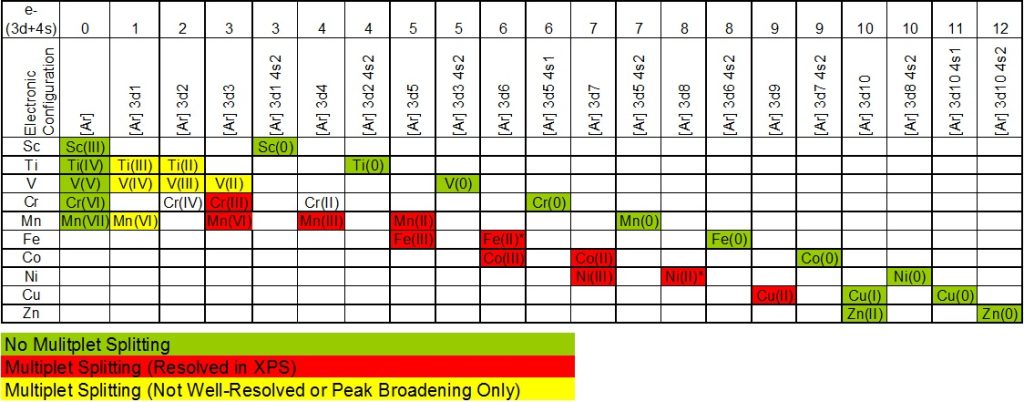
Recall that valence electrons are involved in bonding, so with the valence levels playing a significant role in multiplet splitting, we have a phenomena which is extremely sensitive to the local chemical environment. Such observations are useful, especially for 3d transition metal compounds, to provide fingerprints of metal oxidation states.
3d transition metal compounds exhibiting multiplet splitting are show below (taken from xpsfitting.com)
Further Reading