- Elements
Iron
As with many first row transition metals, analysis of Iron by XPS is non-trivial. The multiplet splittings in the Fe 2p region result in a complex environment which is most easily analysed using a simple fingerprint analysis based on peak energy and shape. Qualitative analysis of the spectra, describing the energy positions of the core lines and satellites is often the most sensible option, where quantitative deconvolutions of multiple oxidation states are either not required or would not be considered reliable due to signal-to-noise or peak overlap complications.
ron Metal
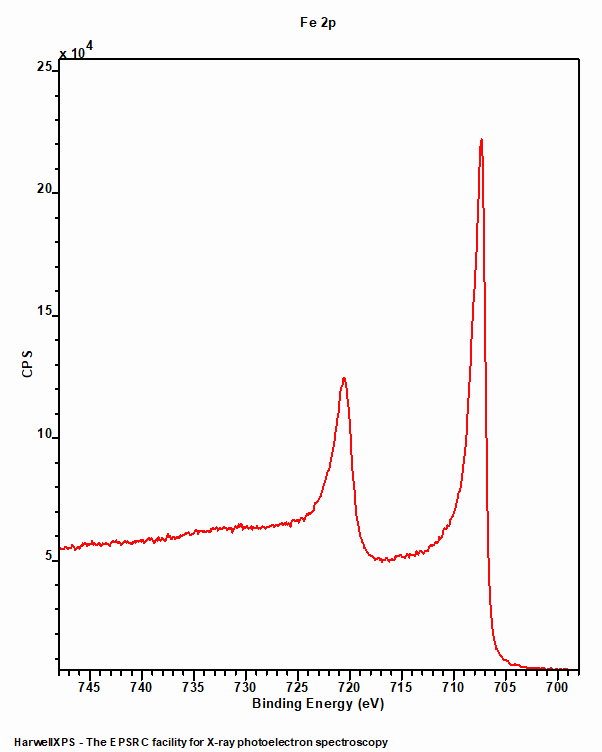
Iron metal (figure 1) exhibits a typical asymmetric peak shape with a doublet consistent with a p-type orbital. The 2p3/2 peak is centred around 706.7 eV and the doublet separation is 13.1 eV.
Low spin Iron compounds
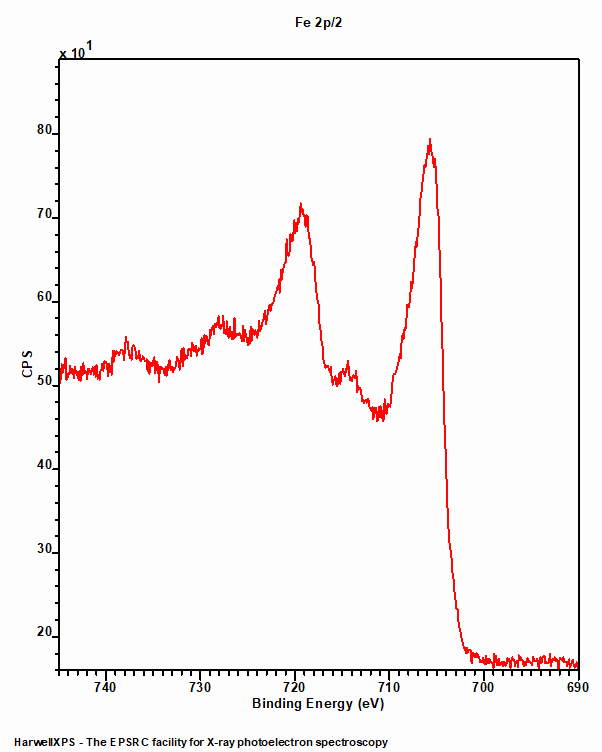
Like Iron metal, low spin Fe compounds do not undergo multiplet splitting and hence exhibit a single photoemission peak (figure 2). Unfortunately, due to the Fe2+ centre requiring a rather large ligand field strength to split the crystal field, such low spin states are not often found amongst the commonly analysed iron compounds such as oxides.

High spin Iron compounds
High spin iron compounds are where things become tricky. Immediately obvious is the increased peak width of the Fe 2p3/2 peak in figure 3. This broadening was found to be largely due to coupling between the 2p core-hole and the unpaired 3d electrons of the photoionized Fe cation, as well as electrostatic and crystal field interactions.(3),(4)
As with any first row TM multiplet (and a general rule for XPS analysis), be careful fitting and only fit what is necessary. Oxidation states may be obtained through a simple process of lineshape analysis – similar to that found in XANES.
If, however, fitting is required, detailed procedures may be found in the work of Biesinger et al.(5)
The intensity of the resultant satellite may be influenced by the identity of the ligand, with increasing electronegativity diminishing the intensity of the satellite – due to a larger barrier for the relaxation process.(7) Furthermore, as electronegativity decreases, as does the energy separation between the satellite and core-line envelope centre. Since a decrease in the electronegativity of the ligand increases the charge density around the metal centre, promoting an electron from the 3d orbital to an unfilled 4s state becomes easier due to the increased shielding and thus the photoelectron loses a smaller amount of kinetic energy during the shake-up process.(8)
Crystal field splitting energy
Grosvernor et al note that the crystal field splitting energy plays an integral role on eventual peak shape, with stronger crystal field spitting ligands causing a greater perturbation from the Gupta and Sen free ion multiplet structure than that observed from weaker crystal field splitting ligands.(9)
References
- Data acquired by HarwellXPS
- Data acquired by HarwellXPS, view it online here, https://doi.org/10.5281/zenodo.4322801
- Gupta, R. and S. Sen (1974). “Calculation of multiplet structure of core p-vacancy levels.” Physical Review B 10(1): 71. Read it online here.
- Gupta, R. and S. Sen (1975). “Calculation of multiplet structure of core p-vacancy levels. II.” Physical Review B 12(1): 15. Read it online here.
- Biesinger, M. C., et al. (2011). “Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni.” Applied Surface Science 257(7): 2717-2730. Read it online here.
- Data acquired by HarwellXPS
- Sarma, D., et al. (1982). “Satellites in the X-ray photoelectron spectra of transition-metal and rare-earth compounds.” Chemical Physics 73(1-2): 71-82. Read it online here.
- Pauling, L. (1960). The Nature of the Chemical Bond, Cornell university press Ithaca, NY. Read it online here.
- Grosvenor, A., et al. (2004). “Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds.” Surface and Interface Analysis: An International Journal devoted to the development and application of techniques for the analysis of surfaces, interfaces and thin films 36(12): 1564-1574. Read it online here.